Interactive Instructions for Use
Use this tool to help determine if BRONCHITOL® (mannitol) inhalation powder is appropriate for your patient
This electronic tool provides step-by-step instructions for performing the BRONCHITOL Tolerance Test (BTT) based on FDA-approved labeling. It will execute calculations routinely done in clinical practice. This tool is not intended to aid clinical decision making, to replace the judgement of a healthcare practitioner, or to perform any clinical assessment. This tool is not intended to replace the Healthcare Practitioner Instructions for Use (HCP IFU) or Patient Information located in the Full Prescribing Information.
The BTT identifies patients who are hyperresponsive to inhaled mannitol, the active ingredient in BRONCHITOL, through a series of monitored inhalations of increasing doses. The BTT must be performed by a healthcare practitioner who is able to manage acute bronchospasm if it were to occur.
STOP the BTT if your patient:
- Has SpO2 or FEV1 measurements that fall below the STOP values calculated in STEP A
- Shows any signs of significant bronchoconstriction requiring treatment with a bronchodilator, such as wheezing or shortness of breath
- Experiences a distressing cough, vomiting, or any other signs that they are not tolerating BRONCHITOL
- Has not inhaled the contents of a total of 10 capsules during STEPS C through F; schedule a repeat BTT
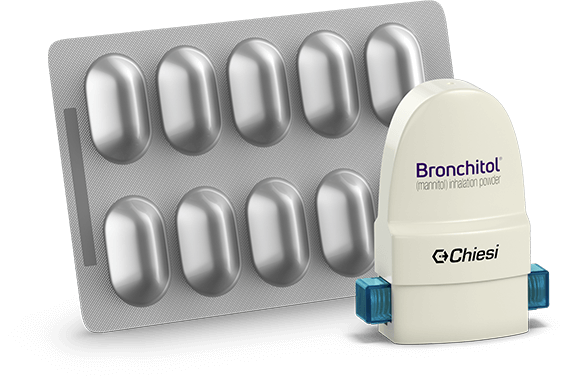
BRONCHITOL Tolerance Test carton includes:
Please ensure you have the following:
- BRONCHITOL Tolerance Test carton
- Bronchodilator (and spacer if needed)
- Timer (one is provided in this tool)
- Spirometer and nose clip
- Pulse oximeter
- Rescue medication and resuscitation equipment
- Sink/hand washing station
- Glass of water for patient to sip during BTT, if necessary
Begin
Step
A
Pre-assessment calculations
Measure baseline SpO2 and FEV1 values. STOP values will be calculated for you.
Today’s baseline
STOP values
SpO2 :
FEV1 :
%x 0.9 = #### %
Lx 0.8 = #### L
90-STOP
(90% of baseline SpO2)
80-STOP
(80% of baseline FEV1)
Step
B
Instruct patient to use an inhaled short-acting beta-agonist.

Wait 5–15 minutes, or use our optional timer:
While waiting, complete these steps:
Please check the boxes above to continue.
Step
C
Following steps 1–8 located below, instruct patient to inhale contents of 1 capsule.
Open inhaler, CONFIRM powder has been inhaled.
Wait 1 minute, or use our optional timer:
Record new SpO2:
%
Step
D
Following steps 3–8 located below, instruct patient to inhale contents of 2 capsules, one capsule at a time.
Open inhaler, CONFIRM powder has been inhaled.
Wait 1 minute, or use our optional timer:
Record new SpO2:
Record new FEV1:
%
L
Step
E
Following steps 3–8 located below, instruct patient to inhale contents of 3 capsules, one capsule at a time.
Open inhaler, CONFIRM powder has been inhaled.
Wait 1 minute, or use our optional timer:
Record new SpO2:
Record new FEV1:
%
L
Step
F
Following steps 3–8 located below, instruct patient to inhale contents of 4 capsules, one capsule at a time.
Open inhaler, CONFIRM powder has been inhaled.
Wait 1 minute, or use our optional timer:
Record new SpO2:
Record new FEV1:
%
L
The patient has passed the BTT.
BRONCHITOL may be prescribed.
Monitor until SpO2 and FEV1 return to baseline.
The recommended dosage of BRONCHITOL is 400 mg twice a day. This requires the inhalation of the contents of 10 capsules (blister pack x 1) twice a day.

The patient has failed the BTT.
DO NOT continue the BTT.
DO NOT prescribe BRONCHITOL.
Wait 15 minutes, then monitor SpO2 and FEV1 to confirm recovery to baseline. Treat bronchospasm as needed.
90-STOP
90% of baseline SpO2
%
80-STOP
80% of baseline FEV1
L
Start
Pause
Continue
Return to main screen
Download Results
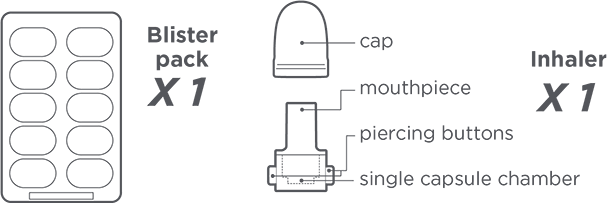
Inhaler use steps for inhalation of the contents of a single capsule
Remove cap.
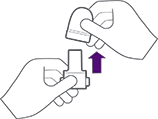
Twist open inhaler by turning the mouthpiece.
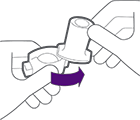
Take 1 capsule out of the package and put it in the chamber.
Do not put the capsule into the mouthpiece.
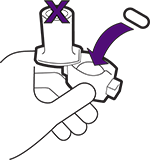
Hold the inhaler upright and turn the mouthpiece until it locks in place.
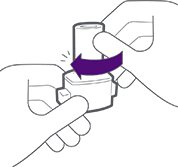
Push both buttons at the same time.
Keep the inhaler upright.
THEN
Release both buttons at the same time.
Never keep buttons pressed.
Exhale fully (do not exhale into inhaler).
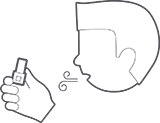
Close lips around mouthpiece and take a steady, deep breath.
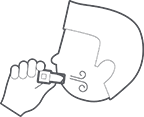
THEN
Remove the inhaler from mouth.
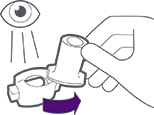
AND
Hold breath for 5 seconds before exhaling (do not exhale into the inhaler).

You should hear a rattling sound while breathing in. If you do not, tap the bottom of the inhaler firmly and repeat steps 6 and 7.
Open the inhaler. If powder is left in the capsule, repeat steps 6 and 7. Once empty, throw the capsule away.
Remove cap.

Twist open inhaler by turning the mouthpiece.

Take 1 capsule out of the package and put it in the chamber.
Do not put the capsule into the mouthpiece.

Hold the inhaler upright and turn the mouthpiece until it locks in place.

Push both buttons at the same time.
Keep the inhaler upright.
THEN
Release both buttons at the same time.
Never keep buttons pressed.
Exhale fully (do not exhale into inhaler).

Close lips around mouthpiece and take a steady, deep breath.

THEN
Remove the inhaler from mouth.

AND
Hold breath for 5 seconds before exhaling (do not exhale into the inhaler).

You should hear a rattling sound while breathing in. If you do not, tap the bottom of the inhaler firmly and repeat steps 6 and 7.
Open the inhaler. If powder is left in the capsule, repeat steps 6 and 7. Once empty, throw the capsule away.

To use this tool please view this page on a desktop or tablet in landscape orientation.
See the Healthcare Practitioner Instructions for Use (HCP IFU) and Full Prescribing Information for more information.
Get an overview about the BRONCHITOL Tolerance Test
Learn more about the BTT
Important Safety
Information
Do not take BRONCHITOL if you have had an allergic reaction to mannitol or any parts of the BRONCHITOL capsule or if you do not pass the BRONCHITOL Tolerance Test (BTT).
Before you take BRONCHITOL, tell your healthcare provider about all your medical conditions, including if you have ever coughed up blood or had blood in your mucus (sputum); are pregnant or planning on becoming pregnant. It is not known if BRONCHITOL will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while using BRONCHITOL; are breastfeeding or plan to breastfeed. It is not known if BRONCHITOL passes into your breast milk or if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby while using BRONCHITOL.
Tell your healthcare provider about all medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
BRONCHITOL may cause serious side effects, including sudden breathing problems immediately after inhaling your medicine; coughing up of blood (hemoptysis). Call your healthcare provider or get emergency medical care right away if you cough up a large amount of blood.
The most common side effects of BRONCHITOL include, cough, coughing up of blood, pain or irritation in the back of your mouth and throat and discomfort when swallowing, vomiting, fever, joint pain, bacteria in your sputum. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of BRONCHITOL. You can ask your healthcare provider or pharmacist for more information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
About BRONCHITOL®
(mannitol) inhalation powder
BRONCHITOL is a prescription medicine that is used along with other therapies to improve lung function in people 18 years of age and older with cystic fibrosis (CF).
BRONCHITOL is only for adults who have passed the BRONCHITOL Tolerance Test (BTT). Your first dose of BRONCHITOL is given during the BTT by your healthcare provider and tests if BRONCHITOL is right for you. Your healthcare provider will use equipment to monitor you and have medicine ready if you have bronchospasm during the test. If you have bronchospasm during your BTT, then you should not be prescribed BRONCHITOL.
BRONCHITOL should not be used in children and adolescents. It is not known if BRONCHITOL is safe and effective in children under 18 years of age.