*The image is not to scale.
For US Healthcare Practitioners
The information provided in this website is intended for US healthcare practitioners only.
I certify that I am a US healthcare practitioner.
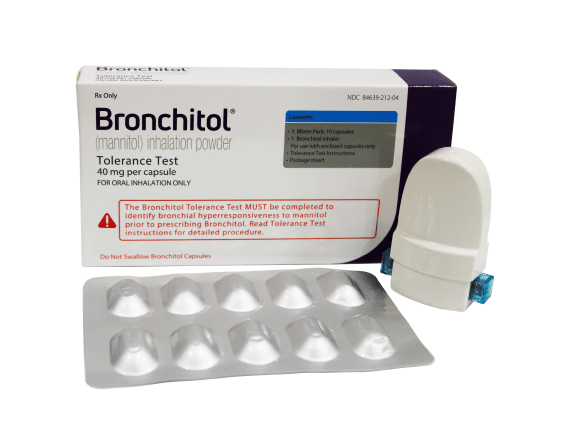
*The image is not to scale.
*The precise mechanism of action of BRONCHITOL in improving pulmonary function in cystic fibrosis (CF) patients is unknown.
BRONCHITOL is not intended to be used as a rescue medication. Inhaler and blister pack are not actual size and are for representation only.Patients prescribed Bronchitol also need to take an inhaled bronchodilator 5 – 15 minutes taking their daily Bronchitol dose (morning and evening).
BRONCHITOL is not intended to be used as a rescue medication. Patients prescribed Bronchitol also need to take an inhaled bronchodilator 5 – 15 minutes taking their daily Bronchitol dose (morning and evening).

Patients taking BRONCHITOL experienced sustained improvements in FEV1 vs control over 26 weeks.1
Review profiles of potential patients for whom BRONCHITOL may be appropriate.

BRONCHITOL is contraindicated in patients with hypersensitivity to mannitol or to any of the capsule components. BRONCHITOL is contraindicated in patients who fail to pass the BRONCHITOL Tolerance Test (BTT).
BRONCHITOL can cause bronchospasm, which can be severe in susceptible patients. Because of the risk of bronchospasm, prior to prescribing BRONCHITOL, patients must pass the BRONCHITOL Tolerance Test (BTT). The BTT must be administered under the supervision of a healthcare practitioner who can treat severe bronchospasm.
Patients who pass the BRONCHITOL tolerance test (BTT) may experience bronchospasm with add-on maintenance therapy with BRONCHITOL. Patients should premedicate with an inhaled short-acting bronchodilator prior to each administration of BRONCHITOL. If bronchospasm occurs, immediately discontinue BRONCHITOL and treat bronchospasm with an inhaled short-acting bronchodilator.
Hemoptysis can occur with BRONCHITOL use. Monitor patients with history of episodes of hemoptysis. If hemoptysis occurs, discontinue use of BRONCHITOL.
Most common adverse reactions (≥3%) include cough, hemoptysis, oropharyngeal pain, vomiting, bacteria sputum identified, pyrexia, and arthralgia.
BRONCHITOL® (mannitol) inhalation powder is a sugar alcohol indicated as add-on maintenance therapy to improve pulmonary function in adult patients 18 years of age and older with cystic fibrosis. Use BRONCHITOL only in adults who have passed the BRONCHITOL Tolerance Test.
Reference: 1. BRONCHITOL® (mannitol) inhalation powder Prescribing Information. 2024.